
Not all products are available in all regions. GDPR Statement | Declaration for California Compliance Law. Any person depicted in such photographs is a model. Photos displayed are for illustrative purposes only.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy.
#ABBOTT ISTAT FDA APPROVAL REGISTRATION#
The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage. This website is governed by applicable U.S. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.

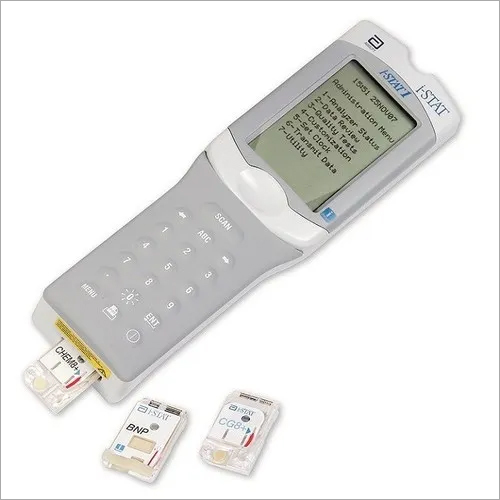
Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. If you have any questions regarding the use of the i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) cartridges with venous and arterial whole blood samples as tests of moderate complexity, please contact your accreditation body.Ībbott Point of Care understands the challenges you are facing during the COVID-19 public health emergency and are fully committed to partnering with you to achieve our shared mission of improving the quality and efficiency of care for the patients we serve.
#ABBOTT ISTAT FDA APPROVAL UPDATE#
If you have any questions regarding this information, please contact your Abbott Point of Care Sales Specialist, Abbott Point of Care Technical Support at 1-84 or via email at or visit CMS will update their publicly posted Frequently Asked Questions (FAQs) in the coming days. NEXT STEPSĭownload and review the i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) cartridge Instructions for Use (IFU) from the Abbott Point of Care website (If you have forwarded any i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) cartridges to another facility, we request that you please provide a copy of this letter to them. Abbott Point of Care will provide additional information regarding the disposition of these tests at the termination of the COVID-19 pandemic. Until the end of the COVID-19 public health emergency, please order the i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) cartridges using the list numbers in the above table. This applies to all i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) cartridges, including those that you may already have on hand.

This is a change from the last customer communication Abbott Point of Care provided to customers on January 15th, 2020. Specifically, during the COVID-19 pandemic, Abbott Point of Care may continue to distribute the i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) cartridges, and facilities that possess a non-waived CLIA certificate (i.e., facilities that hold a Certificate of Compliance or Accreditation) may use them with venous and arterial whole blood samples as tests of moderate complexity. Food and Drug Administration (FDA) and Centers for Medicare and Medicaid Services (CMS) are allowing the use of the i-STAT CG4+ ( blue) and i-STAT G3+ ( blue) blood gas cartridges in laboratories and facilities that hold a Certificate of Compliance or Accreditation to run tests of moderate complexity. We are writing to advise that in light of the COVID-19 public health emergency, the U.S. PH, PO2, PCO2, lactate, TCO 2*, HCO 3*, BE*, sO 2*ĭear Valued Abbott Point of Care Customer,


 0 kommentar(er)
0 kommentar(er)
